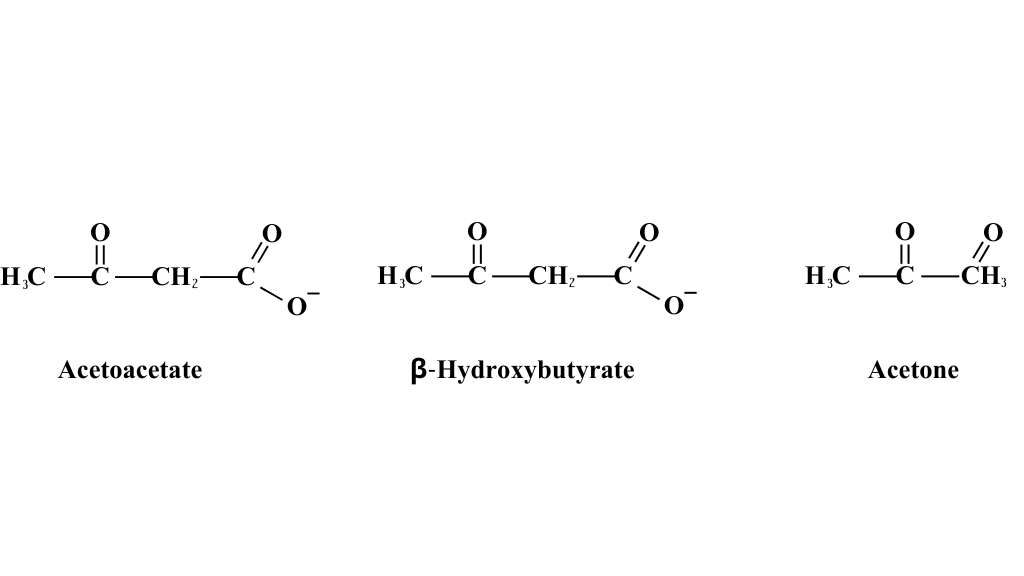
Ketosis a particular metabolic state of the organism to be regarded as a physiological condition that has enabled human beings to survive conditions of famine and prolonged absence of food. Ketosis (i.e., the increase of certain molecules called ketone bodies) can also occur in some pathological conditions, but apart from these conditions, it is to be considered a physiological adaptive response of the organism.
Two methods have historically been used to induce ketosis: either fasting or a diet of meat/fish and fat (such as in the Inuit). Now, on the other hand, it is also possible to implement a ketogenic regimen by using a so-called aglucidic diet that involves foods made to look like carbohydrates while containing no or minimal carbohydrates.
Two methods have historically been used to induce ketosis: either fasting or a diet of meat/fish and fat (such as in the Inuit). Now, on the other hand, it is also possible to implement a ketogenic regimen by using a so-called aglucidic diet that involves foods made to look like carbohydrates while containing no or minimal carbohydrates.